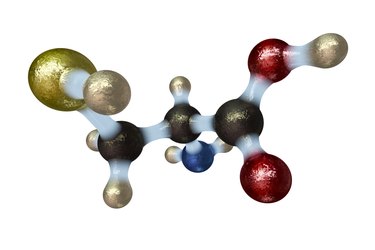
Amino acids are the building blocks for the proteins, enzymes, hormones and neurotransmitters that your body manufactures. All amino acids share a general structure composed of four groups of molecules: a central alpha-carbon with a hydrogen atom, an amine group, a carboxyl group, and a side chain. Your body has 20 different types of amino acids that are identical except for their side chains, which differ in their affinity for water, charge and molecular composition.
General Amino Acid Structure
Video of the Day
Amino acids contain a hydrogen atom; a positively charged amine group, which contains one nitrogen and three hydrogen atoms; and a negatively charged carboxyl group, which contains one carbon and two oxygen atoms. These molecules, along with the variable side chain group, are linked to the central alpha carbon. The positive charge of the amine group is attracted to the negatively charged carboxyl group of other amino acids. The linkage of several of these amino acids forms the backbone of an amino acid chain. This chain folds and interacts with the side chains of other amino acid backbones to form a three-dimensional protein.
Video of the Day
Hydrophobic Amino Acids
A side chain containing a long string of carbons and hydrogens will repel water and form bonds with other water-repelling, or hydrophobic, side chains. This cluster of hydrophobic amino acids creates a stable, physically durable protein structure that does not dissolve in water. Collagen, a structural protein in tissues including your skin, tendons, and ligaments, has a relatively large proportion of hydrophobic amino acids. Conversely, proteins that come in contact with water, such as the hemoglobin in your blood, have a relatively high proportion of hydrophilic, or water-attracting, amino acids.
Amino Acid Charge
The side chains of some amino acids have a positive or negative charge, which helps form a strong interaction with oppositely charged molecules. For example, a binding site for ATP, a negatively-charged molecule, may contain many positively charged amino acids to help facilitate a strong bond to the protein. The electrical charge on these amino acids also makes them water-soluble, which can be essential for proper function. For example, individuals with sickle-cell anemia have a mutation in which one of their hemoglobin protein chains has an uncharged amino acid instead of a negatively charged amino acid. This distorts the shape of the protein, and the red blood cell cannot bind and transport oxygen.
Amino Acids with Sulfur
Cysteine and methionine are the two amino acids that have sulfur in their side chains. The sulfur molecule of a cysteine can bind to the sulfur molecule of another cysteine, creating a strong bond that is hydrophobic and helps maintain the structural integrity of the protein. Keratin, the main structural protein in your hair and fingernails, contains many cysteine amino acids. Although the sulfur molecule in methionine is unable to bind to other sulfurs, it helps facilitate the transfer of molecules in metabolic processes, including protein breakdown and carbohydrate metabolism.